No Products in the Cart
Gaseous or highly volatile toxic chemicals at normal temperature and pressure mainly include ammonia, ozone, nitrogen dioxide, sulfur dioxide, carbon monoxide, carbon dioxide, hydrogen sulfide and photochemical smog. These toxic chemicals mainly come from industrial pollution, the combustion of coal and oil, and the decomposition of biological materials, and these toxic substances have an irritating effect on the respiratory tract and can cause poisoning after inhalation by the human body. Ammonia, ozone, nitrogen dioxide, sulfur dioxide, carbon monoxide, carbon dioxide, hydrogen sulfide, photochemical smog, nitrogen oxides and other atmospheric pollutants are light blue smog formed by photochemical reactions under the action of sunlight, which will cause serious pollution.
Toxic gases are divided into different categories according to different classification standards. Toxic gas can be divided into three categories according to the harm to the human body: nerve paralysis gas, respiratory system paralysis gas, muscle paralysis gas; according to the principle of harm to the human body, it can be divided into irritant gas and suffocating gas. From a medical point of view, asphyxiating gases are toxic gases that can cause hypoxia in the body. Among them, asphyxiating gases can be divided into simple asphyxiating gases, blood asphyxiating gases and cell asphyxiating gases, such as nitrogen, methane, ethane, ethylene, carbon monoxide, and nitro; It is a toxic gas often encountered in the chemical industry; there are many types of irritating gases, the most common are chlorine, ammonia, nitrogen oxides, phosgene, hydrogen fluoride, sulfur dioxide, sulfur trioxide and dimethyl sulfate, etc.
There are many toxic gases that are harmful to human body in life, among which the common toxic gases are carbon monoxide, sulfur dioxide, chlorine, phosgene, diphosgene, Hydrogen Cyanide, Mustard gas, Lewisite, VX (nerve agent), Sarin, BZ (3-Quinuclidinyl benzilate), Tabun, Soman, etc. However, among them, Hydrogen Cyanide, Mustard gas, Lewisite, VX (nerve agent), Sarin, BZ (3-Quinuclidinyl benzilate), Tabun, Soman and other poisonous gases are more toxic. Once leaked, it will cause casualties and huge social effects, so The control of this kind of gas is stricter, and ordinary people have no chance to directly come into contact with this kind of poisonous gas. The toxic gases such as carbon monoxide, sulfur dioxide, chlorine, phosgene, and diphosgene may be exposed to in daily production and living activities, so these toxic gases are more worthy of attention.
Chlorine is a yellow-green gas with a strong pungent odor and a suffocating odor. Many industries and pesticide production are inseparable from chlorine. The harm of chlorine to the human body is mainly manifested in the strong stimulation of the upper respiratory tract mucosa, which can cause respiratory burns, acute pulmonary edema, etc., thereby causing acute lung and heart failure. Acute poisoning is mainly caused by damage to the respiratory system; the onset and condition change rapidly, and there is usually no incubation period. The damage site, nature and extent vary with the inhalation of chlorine gas.
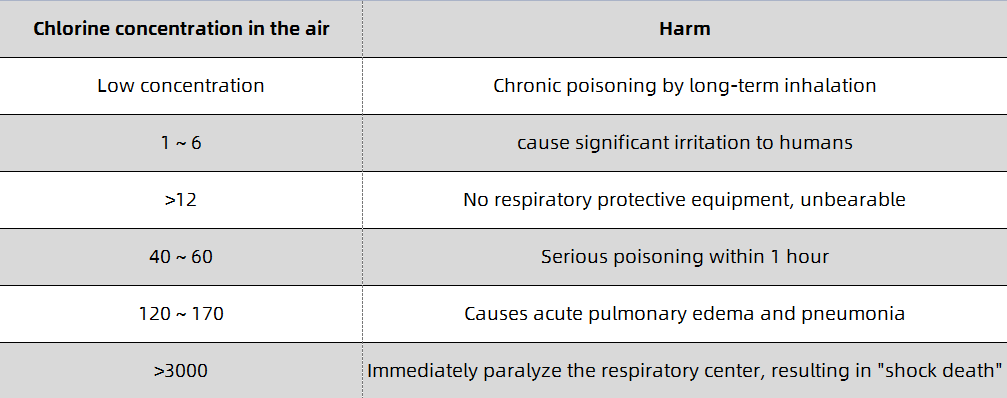
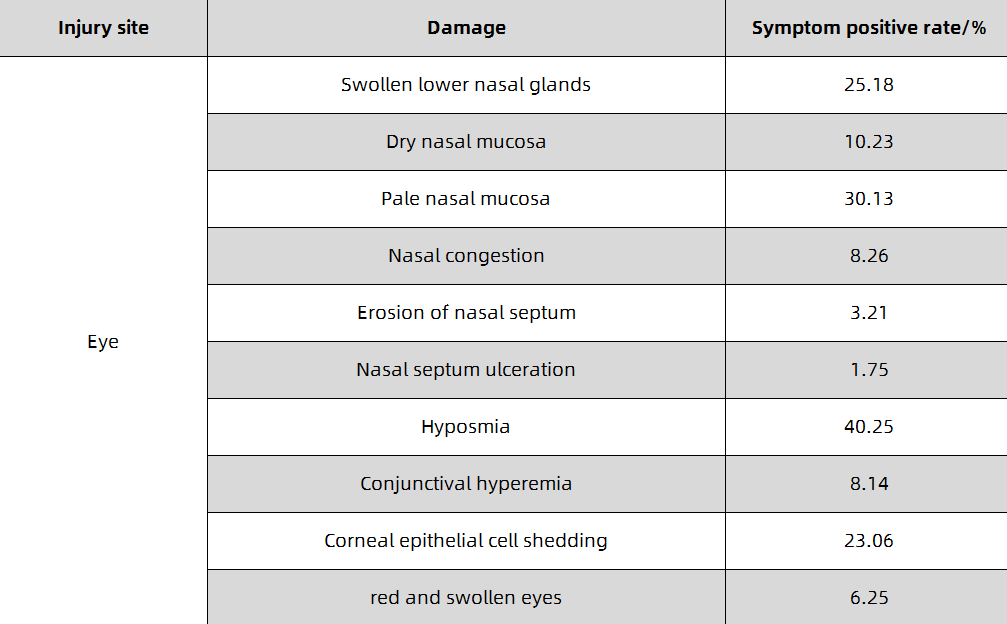
When inhaling a small amount of low-concentration chlorine gas, irritation of the mucosa of the upper respiratory tract may occur, and the symptoms resolve spontaneously within a few hours. When inhaling lower concentrations of chlorine gas, the main manifestations are eye mucous membrane irritation and acute bronchitis, bronchitis or peribronchial inflammation, and the course of the disease is 1-2 days. When inhaling a higher concentration of chlorine gas, the symptoms of lower respiratory tract and pulmonary interstitial changes are generally present, and acute chemical bronchopneumonia, localized alveolar pulmonary edema, interstitial pulmonary edema, and even asthma-like attacks may occur. Lung auscultation, wet and dry sounds or a lot of wheezing, the course of the disease is 1-5 days; when inhaling high concentrations of chlorine, alveolar lesions are generally the main manifestation, and pulmonary edema may appear within 1-2 hours, and a few may appear within 1-2 hours. Happened within 12 hours. Patients present with progressive respiratory rate, dyspnea, cyanotic lips, tachycardia, coughing up white or pink or bloody foamy sputum, refractory hypoxemia, etc., and may even experience coma, cerebral edema or toxic shock. Pulmonary auscultation, wet and dry warm sounds and wheezing sounds, the course of the disease is 1-2 weeks; when inhaling extremely high concentrations of chlorine gas, the peripheral mucosa of the respiratory tract is stimulated by chlorine gas, resulting in local bronchial smooth muscle reflex contractures, and increased ventilation disorders. Respiratory distress symptoms, even laryngospasm and suffocation death, and sometimes vagal reflex cardiac arrest, and electric shock death. It has been reported that bronchial mucosal necrosis and shedding can cause death by asphyxiation. Liquid chlorine or high-concentration chlorine gas can cause acute dermatitis or burns on exposed skin; when the concentration is high, it can cause corneal damage. Different concentrations of chlorine can damage the exposed nose and eyes differently and can cause symptoms to recur. The hazards of different concentrations of chlorine in the air are shown in Table 1-1. The harm of chlorine to the nose and eyes and the positive rate of symptoms are shown in Table 1-2. A small number of cases present with reactive airway insufficiency syndrome (RADS).
Although hydrogen sulfide is colorless, it has an odor similar to rotten eggs. When hydrogen sulfide enters the body through the respiratory tract, it mainly affects the cellular oxidation process, resulting in tissue hypoxia; when a large amount of hydrogen sulfide enters the blood circulation and tissue cells from the alveoli, hydrogen sulfide combines with ferric iron of oxidized cytochrome oxidase, affecting the The process of cellular oxidation, resulting in tissue hypoxia. It affects the nervous system most sensitive to hypoxia first. When hydrogen sulfide comes into contact with the water on the mucous membrane, the hydrogen sulfide dissolves quickly and combines with sodium ions to form sodium sulfide, which has a strong irritating effect on the mucous membranes of the eyes and respiratory tract, causing ophthalmia and even pulmonary edema. In acute poisoning, the symptoms of local irritation are tearing, burning pain in the eyes, photophobia and conjunctival congestion; violent cough, chest distension, nausea and vomiting, dizziness and headache, and with the increase of poisoning, breathing difficulty, panic, blue face, high excitement, mania, and even convulsions, blurred consciousness, and finally coma and general cyanosis will appear.
If exposed to a concentration of 980 ~ 1260mg/m³ for only 15 minutes, the patient will fall into a coma, followed by respiratory paralysis, resulting in death. Studies have shown that hydrogen sulfide can cause damage to the human respiratory system and nervous system. The clinical symptoms are runny nose, dry throat, sore throat, chest pain or chest tightness, severe cough, expectoration, deep and accelerated breathing, and difficulty breathing. Some patients have obvious symptoms. The harm of hydrogen sulfide to the human body is slightly different from that of other toxic gases to the human body, mainly manifested in the effects of different concentrations on the human body. different effects. The harm of low concentration of hydrogen sulfide to the human body is mainly the irritation of the eyes and respiratory tract, while the higher concentration of hydrogen sulfide can cause damage to the human nervous system and suffocation.
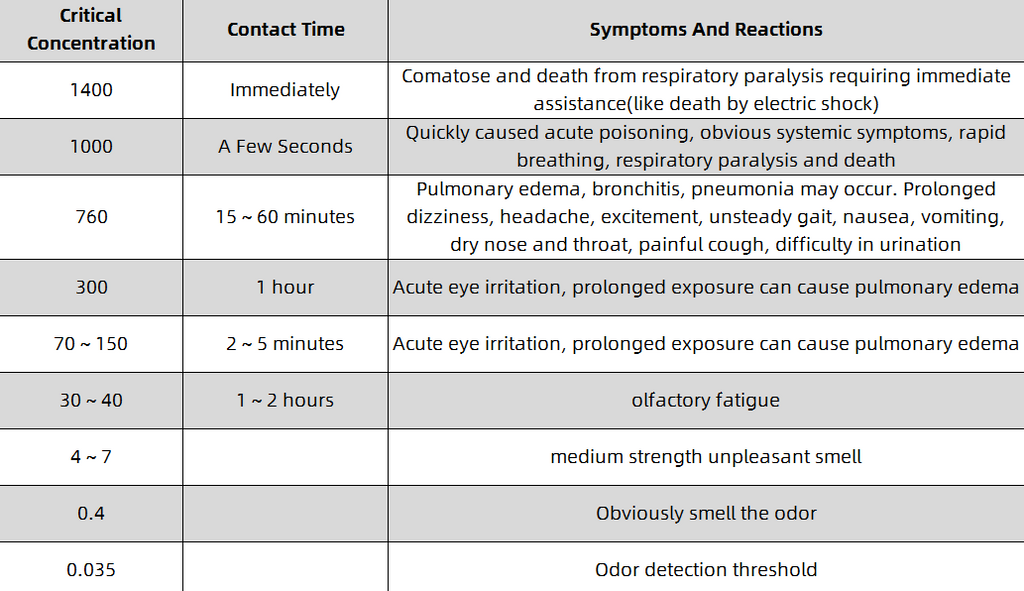
As an acidic substance, hydrogen sulfide can irritate and corrode the mucous membranes of the eyes and respiratory tract. It can also form sodium sulfide with sodium ions on the surface of the mucous membranes, causing symptoms such as eye conjunctivitis and corneal ulcers, and can also cause chemical Bronchitis, toxic pneumonia, and even pulmonary edema. The data shows that when the human body is exposed to an environment with a hydrogen sulfide concentration of 760 mg/m³, fatal injuries can occur in 10 minutes; when exposed to 1000 mg/m³, poisoning can occur in a few seconds, and it will rapidly progress to respiratory paralysis and even death. The human body's olfactory threshold for hydrogen sulfide is 0.012 ~ 0.03mg/m³, and the sense of smell becomes obvious with the increase of the concentration; but when the environmental concentration is > 10mg/m³, the sense of smell will decrease due to olfactory fatigue. Therefore, when a high concentration of hydrogen sulfide occurs in the environment, the human sense of smell will become fatigued, and the risk cannot be detected and judged in a timely and accurate manner. The hazards of hydrogen sulfide to human body are shown in Table 2-1.
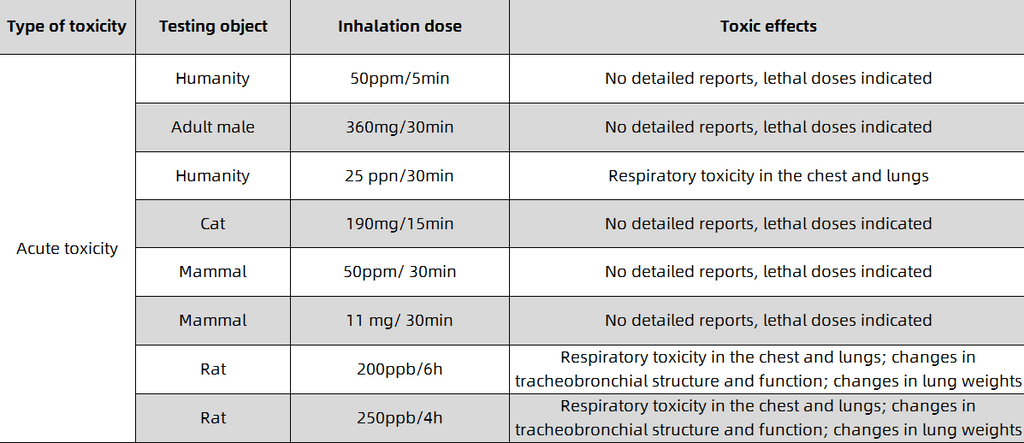
Carbon monoxide, a carbon-oxygen compound with a chemical formula of CO and a relative molecular mass of 28.0101, is usually a colorless, odorless and tasteless gas. In terms of physical properties, carbon monoxide has a melting point of -205°C and a boiling point of -191.5°C. It is insoluble in water and is not easy to liquefy and solidify. In terms of chemical properties, carbon monoxide has both reducing and oxidizing properties, and can undergo oxidation reactions (combustion reactions), disproportionation reactions, etc.; at the same time, it is also toxic. When the concentration of carbon monoxide is high, it can cause different degrees of poisoning symptoms, endanger the brain, heart, liver, kidney, lung and other tissues, and even cause death like electric shock. The minimum lethal concentration of human inhalation is 5000ppm ( 5min).
Industrially, carbon monoxide can be obtained by coke oxygen method and other methods, and is mainly used for the production of methanol and phosgene and organic synthesis. Carbon monoxide poisoning is a product of incomplete combustion of carbon-containing substances, and carbon monoxide enters the human body from the respiratory tract and causes poisoning. The mechanism of carbon monoxide poisoning is that carbon monoxide and oxygen competitively bind to hemoglobin, causing a decrease in blood oxygen concentration.The data show that the affinity of carbon monoxide to hemoglobin is 200-300 times that of oxygen to hemoglobin. When carbon monoxide combines with hemoglobin to form carboxyhemoglobin, hemoglobin loses its ability to carry oxygen and causes tissue stagnation. Therefore, it has toxic effects on the tissues and cells of the whole body, especially on the cerebral cortex.
The severity of carbon monoxide poisoning was positively correlated with the ratio of carboxyhemoglobin (HbCO). When the HbCO saturation is 10% ~ 20%, the clinical manifestations of mild poisoning are headache, dizziness, fatigue, dizziness, dyspnea on exertion; when the Hb-co saturation reaches 30% ~ 40%, the degree of poisoning is significantly aggravated , the patient's lips are cherry red, accompanied by confusion, coma, nausea, vomiting, shock and other symptoms; when the HbCO saturation is greater than 50%, the patient's clinical symptoms continue to aggravate, manifested as deep coma, high fever, muscle tension, paroxysmal Most patients experience myocardial injury, arrhythmia, pulmonary edema, respiratory depression, cerebral edema and even death. Some patients with carbon monoxide poisoning have autonomic neurotrophic disorders, manifested as redness, swelling, blisters, etc. on the skin of the chest or limbs; patients with acute carbon monoxide poisoning often wake up briefly after coma, and have a 2-30-day false recovery period, and then fall into a coma again. In clinical practice, it is called delayed encephalopathy due to carbon monoxide poisoning, which can cause delayed nervous system dysfunction such as tremor, paralysis, dementia, stupor, and sensorimotor disturbance. Data show that long-term exposure to low concentrations of carbon monoxide can cause dizziness, headache, memory loss, difficulty concentrating, palpitations, insomnia and other clinical symptoms.
Sulfur dioxide (S02) is a colorless and transparent gas with a pungent odor, soluble in water, and liquid sulfur dioxide is relatively stable and inactive. Gaseous sulfur dioxide does not decompose or burn when heated to 200°C, and does not form an explosive mixture when mixed with air. Sulfur dioxide is widely used in industry and is a by-product of sulfur ore, papermaking, mineral combustion, and a common pollutant in the atmosphere. Any exposure to higher concentrations: sulfur oxide can cause disease. In addition to directly irritating the eyes and upper airway, the contact with water in the respiratory tract generates sulfuric acid and sulfurous acid, which causes respiratory mucosal damage, which in turn leads to a series of clinical symptoms.
Human exposure to sulphur dioxide can manifest as a biphasic reaction: immediate reactions include irritation and burns to the eyes, nose, and throat, such as conjunctivitis, keratitis, pharyngitis, manifested as sneezing, lacrimation, blurred vision, and tightness in the chest Symptoms, dyspnea, and irritating cough, with rales in the lungs; exposure to high concentrations of sulfur dioxide can cause acute pulmonary edema and death within hours. Some patients who survive in the acute phase may show diffuse pulmonary infiltration or respiratory failure due to persistent airway obstruction 2 to 3 weeks after poisoning. In the atmospheric environment, sulfur dioxide can be oxidized to form sulfate aerosol or sulfuric acid mist, forming acid rain, which is an important substance causing the acidification of the atmospheric environment. The impact of sulfur dioxide on the human body is related to the concentration. When the concentration of sulfur dioxide in the environment is > 0.5ppm, it can cause potential danger to the human body; when the concentration of sulfur dioxide in the environment is 1 to 3ppm, most people can clearly perceive it and cause eye and respiratory tract irritation. Symptoms: When the concentration of sulfur dioxide in the environment is 400 ~ 500ppm, it will cause respiratory mucosal damage, peptic ulcer, and even pulmonary edema, and in severe cases, suffocation and death. Sulphur dioxide and smoke in the air are often superimposed to cause disease, causing damage to the human respiratory tract. When the concentration of sulfur dioxide in the atmospheric environment is > 0.21 ppm, and the concentration of soot is > 0.3 mg/L, the incidence of respiratory tract is often significantly increased, and the condition of patients with chronic diseases accompanied by basic cardiopulmonary diseases deteriorates rapidly. Among the historical events, the London smog event, the Maas Valley smog event, and the Donora smog event are all typical cases caused by the superimposed pathogenic effects of sulfur dioxide and smoke.
Phosgene, also known as phosgene, is highly toxic, non-flammable, and has high chemical reactivity. Solvent, prepared from a mixture of carbon monoxide and chlorine through activated carbon. Phosgene is a colorless gas at room temperature with a rotten grass odor. It is a yellow-green liquid at low temperature. Its chemical properties are unstable. It is rapidly hydrolyzed in water to generate hydrogen chloride. The main damage caused by phosgene inhalation poisoning is toxic pulmonary edema, enlarged pulmonary microvascular space, and enhanced permeability. Increased permeability is the main cause of pulmonary edema caused by diphosgene and phosgene poisoning. There are many studies on the causes of pulmonary edema, such as its acylation, its direct toxic effects, hydrochloric acid effects, nerve reflex effects, and pulmonary hemodynamic changes after poisoning. It is generally believed that the leakage and increased permeability of pulmonary microvascular walls are directly related to the acylation damage of phosgene.
Phosgene is an acyl halide compound, its active group is 0=C (carbonyl group), and its chemical properties are very active. Phosgene can undergo acylation reaction with important functional groups such as amino, sulfhydryl, and hydroxyl groups in proteins in lung tissue cells. , causing extensive inactivation of the respiratory system, which in turn affects the normal metabolism and functional operation of lung tissue cells, damages the lung-qi-blood barrier, increases the permeability of pulmonary capillaries, and eventually leads to pulmonary edema. Secondly, when phosgene is poisoned, the reduction of alveolar surfactant production is also one of the important factors. Under physiological conditions, the surface of the alveoli is secreted by a layer of surfactant, which can reduce the surface tension of the liquid in the alveoli, and is generally secreted by type II alveolar epithelial cells to ensure that the alveoli will not collapse during exhalation, and maintain the balance of water and fluid in the alveoli. . The main component of pulmonary surfactant is dipalmitoyl phosphatidylcholine (DPPC). Acetyl-CoA ester acyltransferase is required for the synthesis of dipalmitoylphosphatidylcholine. After phosgene poisoning, the inactivation of acetyl-CoA ester acyltransferase leads to a decrease in the synthesis of dipalmitoylphosphatidylcholine, its content on the surface of the alveolar wall decreases, the function of DP-PC decreases, and the surface tension of the alveolar liquid increases. Large and large alveoli collapse, the alveolar pressure drops, the pulmonary capillary hydrostatic pressure in equilibrium with it increases, and a large amount of extravasation of fluid in the microvessels enters the pulmonary interstitial tissue, which leads to the occurrence of pulmonary edema.
From the perspective of clinical symptoms, after phosgene inhalation poisoning, there is usually a short-term slowing of respiratory rate, followed by shallow and rapid breathing. After pulmonary edema occurs in the early stage of poisoning, the breathable surface area of the alveoli is progressively reduced, and the alveolar wall is thickened, which will continue to affect the exchange of oxygen and carbon dioxide in the alveoli. Lung ventilation disorder, and eventually circulatory hypoxia, clinical manifestations of continuous decrease in blood oxygen content, progressive increase in CO2 content, and bluish-purple skin superficial mucosa. At this time, the following compensatory changes may occur in the human respiratory and circulatory system, such as deeper and faster breathing, increased activity of the helper muscles, rapid heartbeat, and increased blood pressure. However, in the persistent period of pulmonary edema, the pressure in the lungs increases due to the exudation of fluid in the alveoli, which can continuously increase the load on the right heart; a large amount of plasma components leaks into the space of the lung tissue, which reduces the effective circulating blood volume, intensifies blood dependence, and increases blood pressure. Blood viscosity increases. With the continuous increase of peripheral resistance, the load on the left heart is further increased; severe circulatory hypoxia for a long time will cause myocardial hypoxia, decreased myocardial contractility, and lead to clinical symptoms of heart failure such as arrhythmia and decreased blood pressure. Aggravated tissue hypoxia, forming a vicious circle. With the aggravation of hypoxia, the production of incomplete oxidation products in the body increases, which further leads to the occurrence of acidosis and electrolyte imbalance. When the blood CO2 content gradually decreases, visceral capillaries dilate, peripheral capillaries constrict, skin and mucous membranes turn pale, blood pressure drops sharply, acute circulatory failure may occur, and the body enters a state of physical restraint. At the end stage, pulmonary edema is combined with circulatory failure, and the body completely loses its ability to compensate. With the further development of pulmonary edema, a large amount of plasma extravasates from the brain capillaries, resulting in a decrease in plasma volume, blood concentration, and a decrease in plasma protein, and the number of red and white blood cells. Hemoglobin increased and hematocrit increased, and these changes were consistent with the degree of pulmonary edema. Due to the thick blood, slow blood flow, and tissue damage, blood coagulation increases, resulting in thrombosis and peripheral circulation embolism. Because the central nervous system is very sensitive to hypoxia, the cerebral cortex appears transiently excited in the early stage of hypoxia, causing headache, dizziness, and irritability; as the degree of hypoxia increases, the brain gradually enters a state of inhibition, manifested as indifferent expression, fatigue, etc.; The development of hypoxia can make the inhibition of the cerebral cortex continue to increase and spread to the subcortical layer. The respiratory center of the medulla oblongata can change from excitation to inhibition, and breathing and heartbeat are weakened, and even central paralysis occurs, and even death occurs due to respiratory and heartbeat arrest. The toxic effects of phosgene on organisms are shown in Table 5-1.
Nerve agent is a kind of chemical weapon. Compared with other chemical weapons (such as mustard gas, chlorine gas, etc.), nerve agent can damage the human nervous system in a short period of time and cause damage to living beings, usually without affecting other tissues of the human body. Nerve agent is one of the earliest chemical agents used in warfare. As early as World War I, the German army began to use hydrogen on the battlefield to kill the British and French forces. But it wasn't until the 1930s that the world's first nerve agent, GA (tabun), was successfully developed by German scientists at Farben. Until now, due to the high lethality, simple synthesis and convenient use of nerve agents, they still attract many irregular armed forces, terrorist organizations and "jackax" terrorists to use them in conflicts. Now, according to various organizations (such as NATO) and the US military), the most commonly used nerve agents are still G series (such as sarin and tabun) and V series (such as VX) developed in the 1930s and 1960s, and their chemical structures and mechanisms of action Also highly similar.
There are two main types of nerve agents. The members of these two groups have similar properties and common names. The North Atlantic Treaty Organization is marked with two English letters, namely the G series and the V series. The G-series is the first and oldest family of nerve agents and is non-persistent; while all V-series nerve agents are persistent, meaning that these agents are not easily broken down or washed off and can remain in place for long periods of time. clothes and surfaces. Nerve agents can bind to acetylcholinesterase (acetylcholinesterase has extremely high hydrolytic activity, one molecule of acetylcholinesterase can hydrolyze 25,000 molecules of acetylcholine per second). The two substances are combined by chemical bonds, and the combined acetylcholinesterase can no longer play a physiological role, and because the combination of the nerve agent and the enzyme cannot be reversed by any physiological mechanism, unless the human body synthesizes a new enzyme, otherwise, during this period of time, Acetylcholine (a neurotransmitter, the substance responsible for transmitting information between nerve cells) at this site cannot be recycled normally, but diffuses randomly to stimulate the nervous system, causing a series of neurological disorders and even death.
To sum up, nerve agents are targeted by the killing of neurotransmitters by toxic compounds. The representatives of this poisonous gas are chlorine gas during World War I and Tabun (GA) and Sarin (GB) which were not used in World War II. However, because it is regulated by the United Nations Convention, it is not used in regular wars. Only in the terrorist activities of some terrorist organizations have nerve agents been used several times. For example, in the Syrian war, the "lone wolf" was used. Tokyo subway in Japan horrific events that occurred. According to the British Daily Mail in 2013, the suburbs of the Syrian capital Damascus were bombarded with nerve gas by government forces in August, killing 1,300 sleeping people, including a large number of women and children. According to a follow-up investigation by the United Nations, it was found that the poison gas in the rocket was the sarin gas in the nerve agent.
The harm of nerve paralysis poisonous gas to human body
Due to the particularity of the nerve-paralyzing poisonous gas, generally only those who have encountered the poisonous agent on the field and who have encountered a similar Tokyo subway incident will have the possibility to come into contact with them. According to relevant statistics, in the 1980s, thousands of Iranians died due to exposure to the nerve agents sarin and tobin released by the Iraqi army. , but due to the lack of protective equipment and the high adhesion of such agents, American soldiers suffered certain sequelae. Among them, more than 10,000 American soldiers who were exposed to these poisonous gases returned home with malaise, limb pain, memory loss, and intermittent diarrhea and superficial lumps. Because these soldiers had experienced the Gulf War, so this kind of symptoms Also known as Gulf Symptom Syndrome.
When respiratory paralysis gas poisoning occurs, the symptoms are dizziness, nausea, vomiting, coma, and some of the poisoning symptoms of respiratory paralysis gas are skin ulceration and tracheal mucosal ulceration. Deep poisoning of these gases can lead to shock and even death. At the same time, the respiratory system paralyzing gas is also used in the fields of pesticides and various pharmaceuticals. Among them, the respiratory system paralysis gas is the gas that causes damage to the respiratory system by acting on the respiratory system. In industrial production, the respiratory tract is most likely to be exposed to poisons, especially irritating poisons. Once inhaled, it can cause breathing difficulties in mild cases and chemical pneumonia or pulmonary edema in severe cases. Respiratory paralysis poisons that cause respiratory damage include chlorine, ammonia, sulfur dioxide, phosgene, and nitrogen oxides. Acute inhalation of irritating toxins can cause rhinitis, laryngitis, glottis edema, tracheobronchitis, etc. The symptoms include runny nose, sneezing, sore throat, expectoration, chest pain, shortness of breath, and difficulty breathing, which can easily cause chemical pneumonia. It causes inflammation of the lungs, which is more harmful to the human body than acute respiratory tract inflammation. Patients will have severe cough, expectoration (sometimes bloodshot in the sputum), chest tightness, chest pain, shortness of breath, difficulty breathing, and fever. In patients with chemical pulmonary edema, the alveoli and between the alveoli are filled with fluid, which is mostly caused by inhaling a large amount of irritating gas. It is the most serious respiratory disease. If the rescue is not timely, it can cause death. The patient has obvious dyspnea, bruising of skin and mucous membranes, severe cough, with a lot of pink phlegm, irritability and other symptoms. Chronic bronchitis can be caused by long-term inhalation of low-concentration irritating gas or dust. cause emphysema.